Plastics are useful and ubiquitous in our society. However, they also raise environmental problems due to their persistence in nature. Thus, sustainable approaches to the valorization of plastic waste remain of utmost importance. In the Stache lab, we are developing mild photooxidative degradation methods to upcycle post-consumer plastic waste into commercially important chemical feedstocks. In this method, an inexpensive oxidant (molecular O2), visible light, and nontoxic iron based photocatalysts are used.
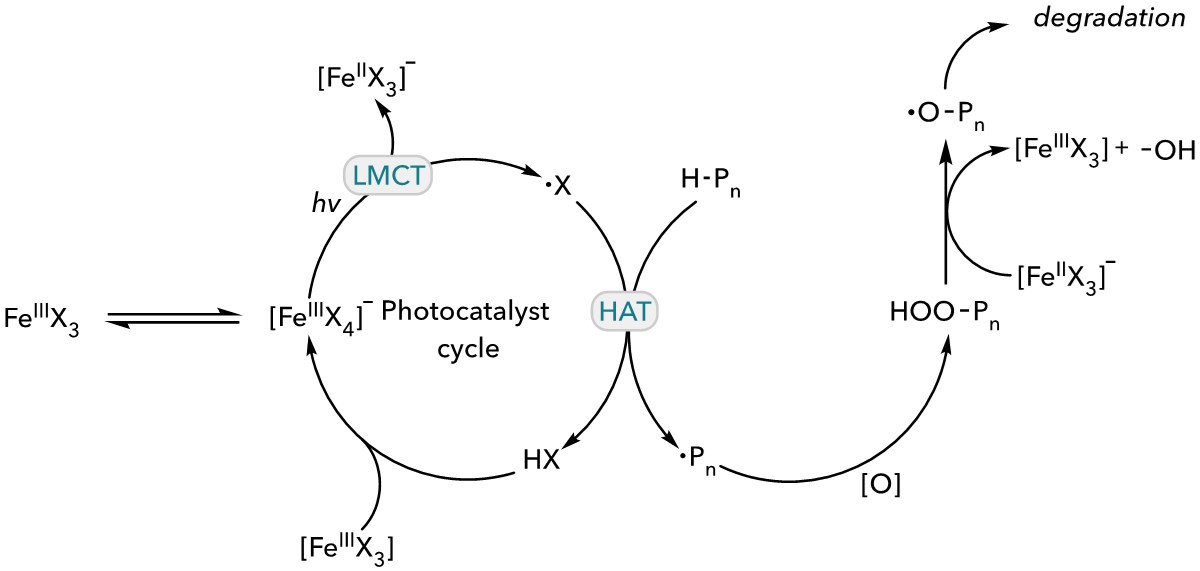
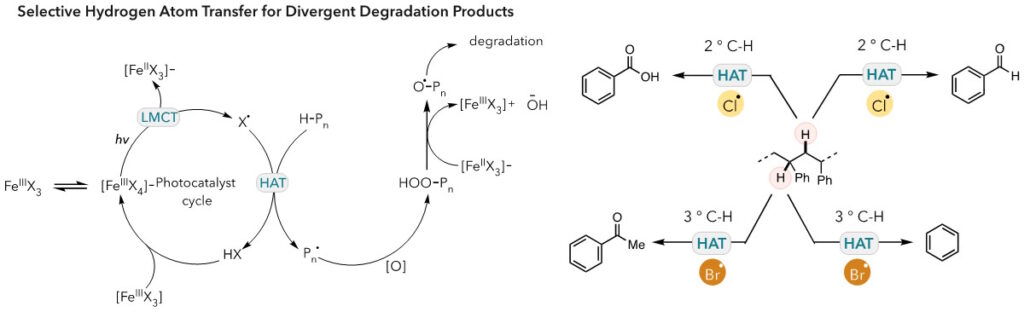
During the photooxidative degradation mechanism, FeCl3 or FeBr3 undergo disproportionation to form [FeIIIX4]– anion species. Upon the irradiation of light, halogen radical is generation through ligand-to-metal charge transfer (LMCT). The halogen radical performs hydrogen atom transfer (HAT) on polymer backbone to form HX and a carbon-centered radical on polymer. The resulting radical is quenched by molecular O2 to form hydroperoxyl group, which then cleaved to an oxygen-centered radical with an assistance of [FeIIX3]– through Fenton-like chemistry. The oxygen-centered radical on polymer backbone enables β-chain scission to cleave C–C bond on polymer backbone. Lastly, HX and [FeIIIX3] recombine to close the photocatalyst cycle.
We initially chose to study photooxidative degradation of polystyrene. Through a choice of photocatalyst, the product distribution of polystyrene degradation could be altered to favor different products. In the case of a chlorine radical, polystyrene was converted to benzoic acid. Yet, the polystyrene degradation through a bromine radical, a more thermoneutral process, showed that benzoic acid and acetophenone were generated in equal measure, showing a shift in product distribution. We are continuing to investigate these reactivity-selectivity relationships. We will also apply this method to other class of polymers, develop new catalysts or new system to obtain different types of products, and repurpose the degraded polymers or oligomers for making new materials.
