Advancing materials science and synthesis.
Based in the Frick Chemistry Laboratory at Princeton University, the Stache Lab integrates diverse chemistry disciplines, including organic chemistry, photochemistry, inorganic materials, and polymer chemistry, to pioneer advancements in materials science and synthesis.
Recent publications.
-
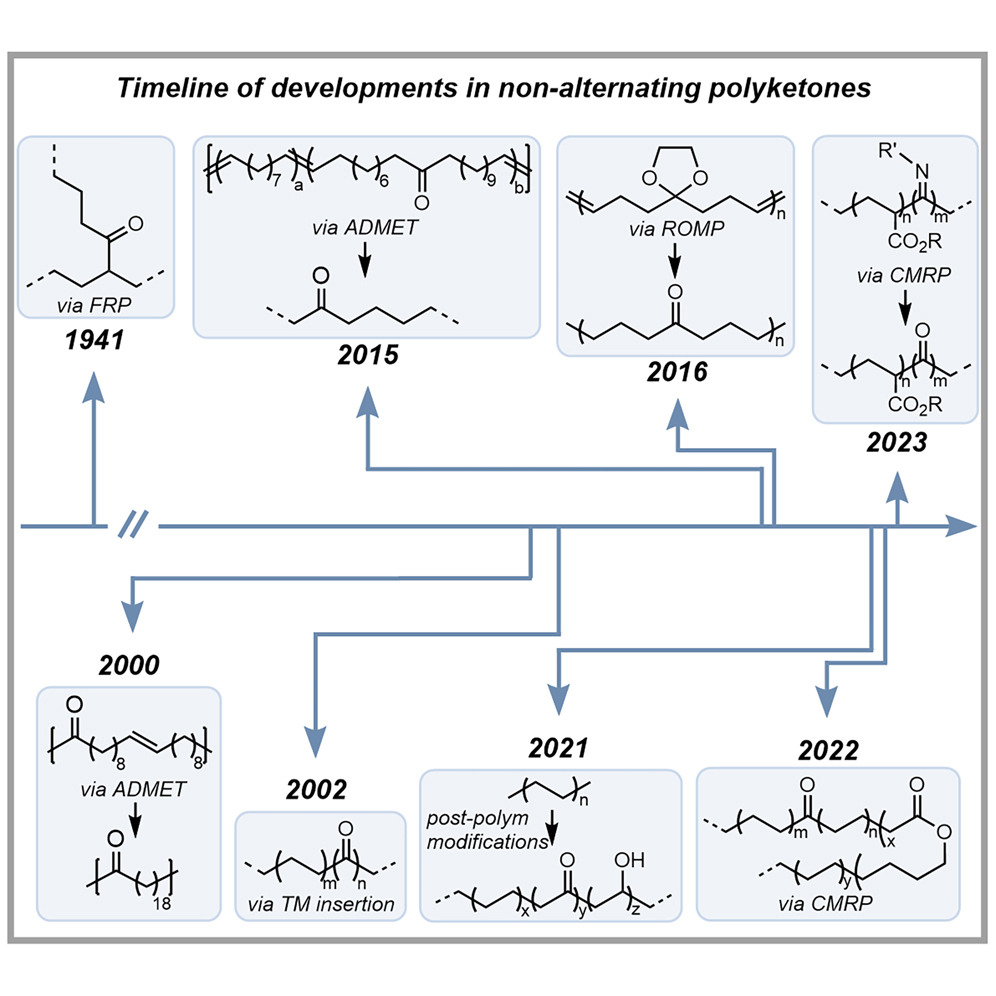
An old polymer class revisited: Versatile, degradable, non-alternating polyketones
Publication Abstract
Commercial plastics are essential to our society; however, they persist in the environment due to their chemical inertness. Plastic pollution causes serious environmental problems, necessitating new, sustainable strategies to break them down. Developing degradable materials that will decompose in the environment is of immense interest. However, these materials must also be of commercial relevance, and easily degradable polymers often do not display the necessary physical properties. This perspective will highlight one of these polymer classes: non-alternating polyketones. Historical perspective on the original synthesis and recent advances for synthesizing these materials highlight the longstanding interest in these polymers. Additionally, post-polymerization modifications and photodegradation studies will provide context for the potential applications of these interesting polymers.
-
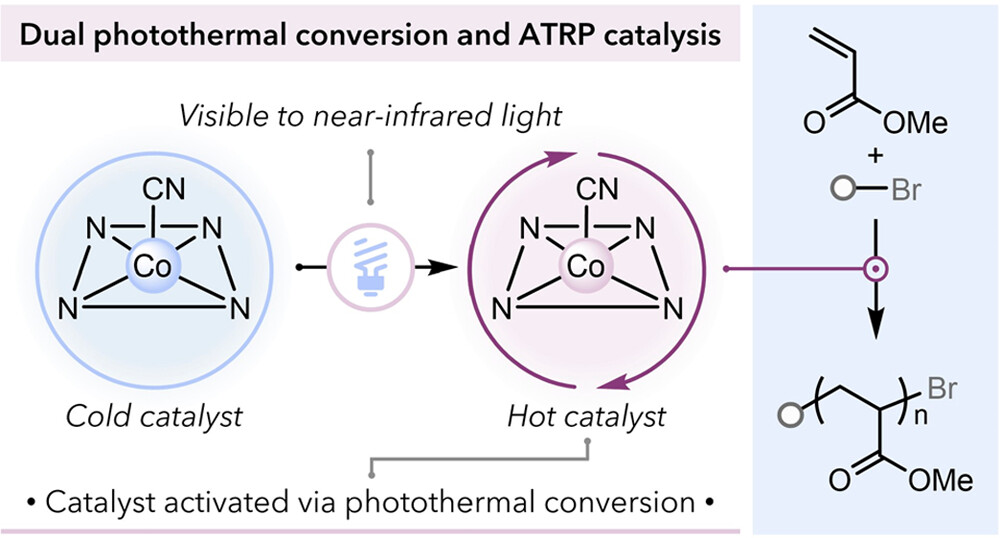
Molecular Photothermal Conversion Catalyst Promotes Photocontrolled Atom Transfer Radical Polymerization
J. Am. Chem. Soc. 2024, 146, 8852-8857
Publication Abstract
Photothermal conversion is a growing research area that promotes thermal transformations with visible light irradiation. However, few examples of dual photothermal conversion and catalysis limit the power of this phenomenon. Here, we take inspiration from nature’s ability to use porphyrinic compounds for nonradiative relaxation to convert light into heat to facilitate thermal polymerization catalysis. We identify the photothermal conversion catalytic activity of a vitamin B12 derivative, heptamethyl ester cobyrinate (HME-Cob), to perform atom transfer radical polymerization (ATRP) under irradiation. Rapid polymerization are obtained under photothermal activation while maintaining good control over polymerization with the aid of a photoinitiator to enable light-induced catalyst regeneration. The catalyst exhibits exquisite temporal control in photocontrolled thermal polymerization. Ultimately, the activation of this complex is accessed across a broad range of wavelengths, including near-IR light, with excellent temporal control. This work showcases the potential of developing photothermal conversion catalysts.
-

Selective poly(vinyl ether) upcycling via photooxidative degradation with visible light
Chem. Sci. 2024, 15, 1840-1845
Publication Abstract
Poly(vinyl ethers) (PVEs) have many applications, such as adhesives, lubricants, and anticorrosive agents, thanks to their elastic, nonirritating, and chemically inert properties. The recycling of PVEs remains largely underexplored, and current methods lack generality towards other polymer classes. Thus, the chemical upcycling of PVE into small molecule feedstocks would provide an alternative approach to combat these current issues. Here, we report a visible light-mediated method of upcycling poly(isobutyl vinyl ether) (PIBVE) into small molecules via photooxidative degradation using chlorine or bromine radicals. PIBVE can be degraded to low molecular weight oligomers within 2 h, producing good yields of alcohols, aldehydes, and carboxylic acids. Mechanistic studies suggest that hydrogen atom transfer (HAT) from the backbone or the side chain leads to small molecule generation via oxidative cleavages. Additionally, this protocol was applied to a copolymer of poly(methyl acrylate-co-isobutyl vinyl ether) to demonstrate the preference for the degradation of polymers bearing more electron-rich C–H bonds through a judicious choice of abstraction agent. Ultimately, we show that photooxidative degradation enables the selective chemical upcycling of PVEs as a method of plastic waste valorization.

Our team.
The Stache Lab is led by Principal Investigator, Erin Stache. Our research team comprises a group of exceptional post-doctoral associates and graduate students.

