-
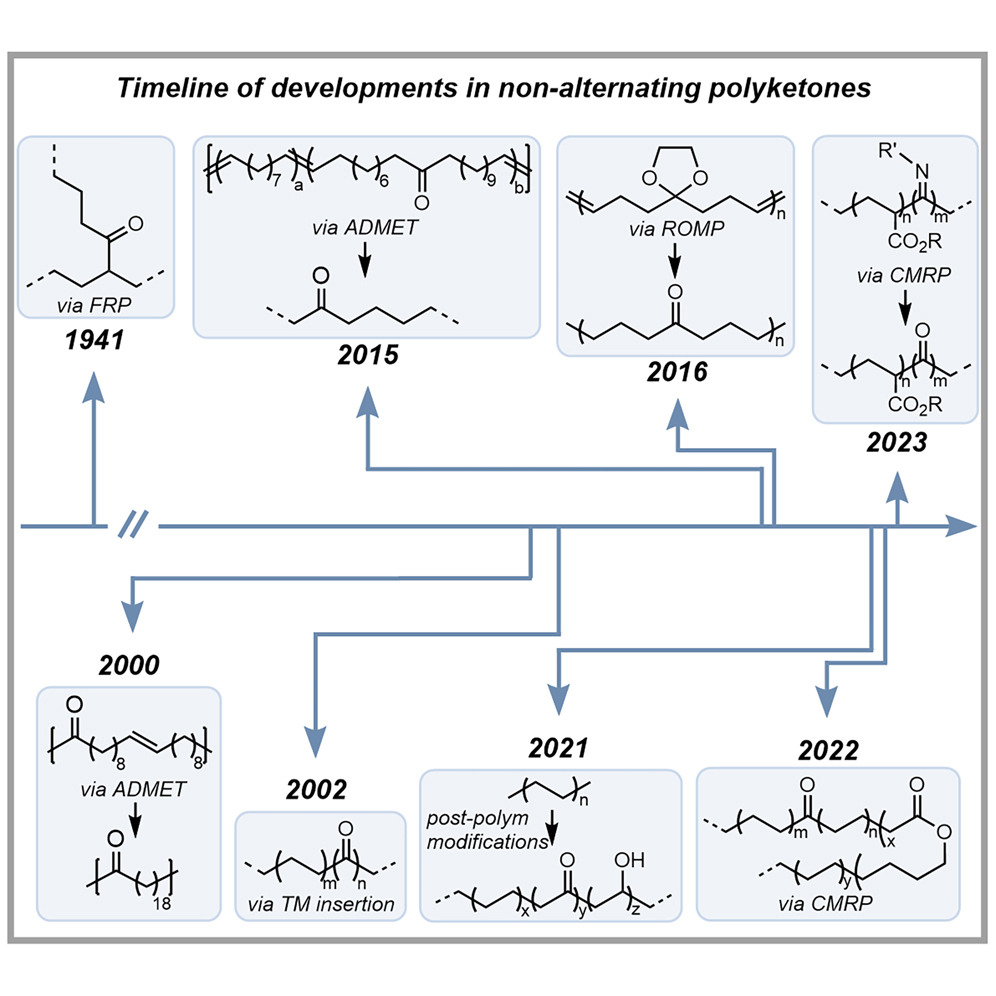
An old polymer class revisited: Versatile, degradable, non-alternating polyketones
Publication Abstract
Commercial plastics are essential to our society; however, they persist in the environment due to their chemical inertness. Plastic pollution causes serious environmental problems, necessitating new, sustainable strategies to break them down. Developing degradable materials that will decompose in the environment is of immense interest. However, these materials must also be of commercial relevance, and easily degradable polymers often do not display the necessary physical properties. This perspective will highlight one of these polymer classes: non-alternating polyketones. Historical perspective on the original synthesis and recent advances for synthesizing these materials highlight the longstanding interest in these polymers. Additionally, post-polymerization modifications and photodegradation studies will provide context for the potential applications of these interesting polymers.
-
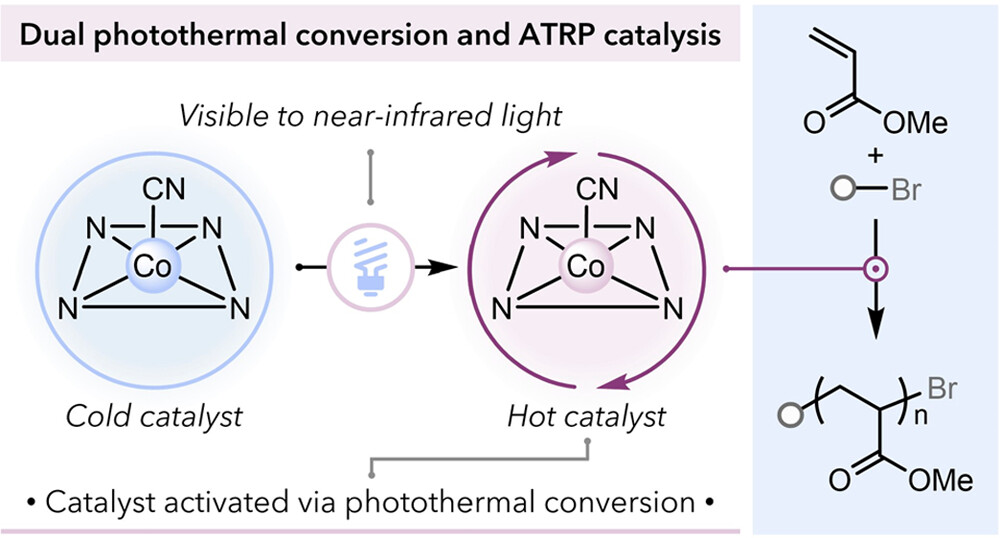
Molecular Photothermal Conversion Catalyst Promotes Photocontrolled Atom Transfer Radical Polymerization
J. Am. Chem. Soc. 2024, 146, 8852-8857
Publication Abstract
Photothermal conversion is a growing research area that promotes thermal transformations with visible light irradiation. However, few examples of dual photothermal conversion and catalysis limit the power of this phenomenon. Here, we take inspiration from nature’s ability to use porphyrinic compounds for nonradiative relaxation to convert light into heat to facilitate thermal polymerization catalysis. We identify the photothermal conversion catalytic activity of a vitamin B12 derivative, heptamethyl ester cobyrinate (HME-Cob), to perform atom transfer radical polymerization (ATRP) under irradiation. Rapid polymerization are obtained under photothermal activation while maintaining good control over polymerization with the aid of a photoinitiator to enable light-induced catalyst regeneration. The catalyst exhibits exquisite temporal control in photocontrolled thermal polymerization. Ultimately, the activation of this complex is accessed across a broad range of wavelengths, including near-IR light, with excellent temporal control. This work showcases the potential of developing photothermal conversion catalysts.
-

Selective poly(vinyl ether) upcycling via photooxidative degradation with visible light
Chem. Sci. 2024, 15, 1840-1845
Publication Abstract
Poly(vinyl ethers) (PVEs) have many applications, such as adhesives, lubricants, and anticorrosive agents, thanks to their elastic, nonirritating, and chemically inert properties. The recycling of PVEs remains largely underexplored, and current methods lack generality towards other polymer classes. Thus, the chemical upcycling of PVE into small molecule feedstocks would provide an alternative approach to combat these current issues. Here, we report a visible light-mediated method of upcycling poly(isobutyl vinyl ether) (PIBVE) into small molecules via photooxidative degradation using chlorine or bromine radicals. PIBVE can be degraded to low molecular weight oligomers within 2 h, producing good yields of alcohols, aldehydes, and carboxylic acids. Mechanistic studies suggest that hydrogen atom transfer (HAT) from the backbone or the side chain leads to small molecule generation via oxidative cleavages. Additionally, this protocol was applied to a copolymer of poly(methyl acrylate-co-isobutyl vinyl ether) to demonstrate the preference for the degradation of polymers bearing more electron-rich C–H bonds through a judicious choice of abstraction agent. Ultimately, we show that photooxidative degradation enables the selective chemical upcycling of PVEs as a method of plastic waste valorization.
-
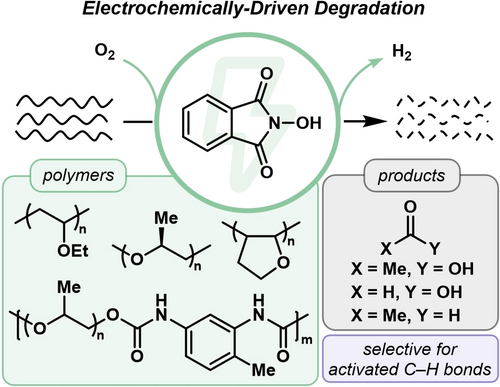
Selective Electrocatalytic Degradation of Ether-Containing Polymers
Angew. Chem. Int. Ed. 2023, e202316578
Publication Abstract
Leveraging electrochemistry to degrade robust polymeric materials has the potential to impact society’s growing issue of plastic waste. Herein, we develop an electrocatalytic oxidative degradation of polyethers and poly(vinyl ethers) via electrochemically mediated hydrogen atom transfer (HAT) followed by oxidative polymer degradation promoted by molecular oxygen. We investigated the selectivity and efficiency of this method, finding our conditions to be highly selective for polymers with hydridic, electron-rich C−H bonds. We leveraged this reactivity to degrade polyethers and poly(vinyl ethers) in the presence of polymethacrylates and polyacrylates with complete selectivity. Furthermore, this method made polyacrylates degradable by incorporation of ether units into the polymer backbone. We quantified degradation products, identifying up to 36 mol % of defined oxidation products, including acetic acid, formic acid, and acetaldehyde, and we extended this method to degrade a polyether-based polyurethane in a green solvent. This work demonstrates a facile, electrochemically-driven route to degrade polymers containing ether functionalities.
-
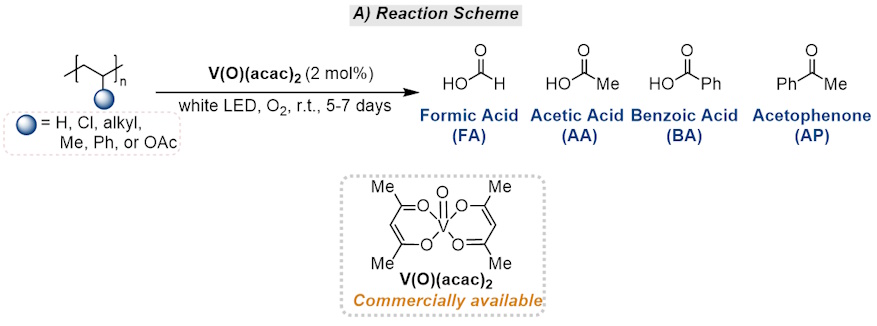
From Trash to Treasure: Vanadium-Catalyzed Upcycling of Non-Biodegradable Plastics
Publication Abstract
Managing plastic waste is a challenging and pressing problem. In this issue of Chem, Soo and co-workers valorize commercial plastics and post-consumer plastic waste into isolable products, including formic acid, acetic acid, and benzoic acid, through vanadium-catalyzed tandem C–H oxidation and C–C cleavage photoreactions.
-
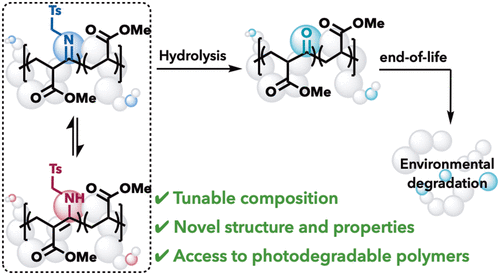
Controlled Radical Polymerization of Acrylates and Isocyanides Installs Degradable Functionality into Novel Copolymers
J. Am. Chem. Soc. 2023, 145, 37, 20311–20318
Publication Abstract
Installing ketones into a polymer backbone is a known method for introducing photodegradability into polymers; however, most current methods are limited to ethylene–carbon monoxide copolymerization. Here we use isocyanides in place of carbon monoxide in a copolymerization strategy to access degradable nonalternating poly(ketones) that either maintain or enhance the thermal properties. A cobalt-mediated radical polymerization of acrylates and isocyanides synthesizes nonalternating poly(acrylate-co-isocyanide) copolymers with tunable incorporation using monomer feed ratios. The kinetic product of the polymerization is a dynamic β-imine ester that tautomerizes to the β-enamine ester. Hydrolysis of this copolymer affords a third copolymer microstructure─the elusive nonalternating poly(ketone)─from a single copolymerization strategy. Analysis of the copolymer properties demonstrates tunable thermal properties with the degree of incorporation. Finally, we show that poly(acrylate-co-isocyanide) and poly(acrylate-co-ketone) are photodegradable with 390 nm light, enabling chain cleavage.
-
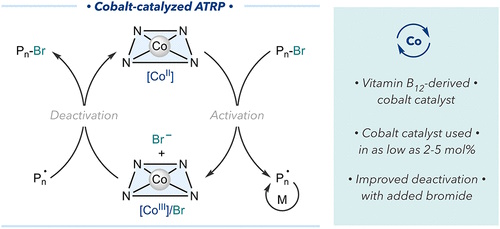
Vitamin B12 Derivative Enables Cobalt-Catalyzed Atom Transfer Radical Polymerization
J. Am. Chem. Soc. 2023, 145, 35, 19387–19395
Publication Abstract
Advances in controlled radical polymerizations by cobalt complexes have primarily taken advantage of the reactivity of cobalt as a persistent radical to reversibly deactivate propagating chains by forming a carbon–cobalt bond. However, cobalt-mediated radical polymerizations require stoichiometric ratios of a cobalt complex, deterring its utility in synthesizing well-defined polymers. Here, we developed a strategy to use cobalt as a catalyst to control radical polymerizations via halogen atom transfer with alkyl halide initiators. Using a modified, hydrophobic analogue of vitamin B12 (heptamethyl ester cobyrinate) as a cobalt precatalyst, we controlled the polymerization of acrylate monomers. The polymerization efficiency of the cobalt catalyst was significantly improved by additional bromide anions, which enhanced the deactivation of propagating radicals yielding polymers with dispersity values <1.2 using catalyst concentrations as low as 5 mol %. We anticipate that the development of cobalt catalysis in atom transfer radical polymerization will enable new opportunities in designing catalytic systems for the controlled synthesis of polymers.
-
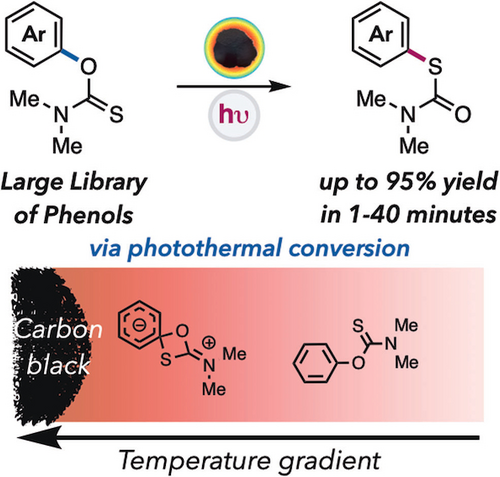
Photothermal Conversion by Carbon Black Facilitates Aryl Migration by Photon‐Promoted Temperature Gradients
Angew. Chem. Int. Ed. 2023, e202308648
Publication Abstract
The Newman Kwart Rearrangement (NKR) offers an efficient and high-yielding method for producing substituted thiophenols from phenols. While an industrially important protocol, it suffers from high activation energy barriers (35-43 kcal/mol), requiring the use of extreme temperatures (>200 °C) and specialty equipment. This report details a highly efficient and straightforward method for facilitating the NKR using photothermal conversion. This underused, unique reactivity pathway arises from the irradiation of nanomaterials that relax via a non-radiative decay pathway to generate intense thermal gradients. We show carbon black (CB) can be an inexpensive and abundant photothermal agent under visible light irradiation to achieve a facile NKR under mild conditions. The scope includes a wide array of stereo- and electronically diverse substrates with increasing difficulty of rearrangement, including BHT and BINOL as effective substrates. Furthermore, we demonstrate the unique application for temporal control in a thermal reaction and tunability of thermal gradients by modulating light intensity. Ultimately, photothermal conversion enables high-temperature reactions with simple, visible light irradiation.
-
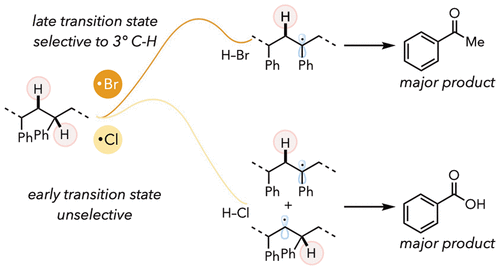
Mechanistic insights enable divergent product selectivity in catalyst-controlled photooxidative degradation of polystyrene
ACS Catal. 2023, 13, 16, 10968–10975
Publication Abstract
Polystyrene upcycling to valuable commodity feedstocks is essential for reducing plastic waste. Photooxidative degradation has emerged as a method for converting polystyrene to oxidized aromatic compounds. Investigating the mechanism of photooxidative degradation is essential for understanding the pathways to generating different small molecules. Here, we leveraged the reactivity differences between chlorine and bromine radicals to study the degradation mechanism of polystyrene. While degradation with chlorine radical yields primarily benzoic acid (50 mol %), bromine radical shows an increased preference for acetophenone (4 mol % for both products). After conducting several mechanistic studies, we discovered that activation of 3° C–H bonds is necessary to form acetophenone. However, for acetophenone production, a second hydrogen atom transfer from H–Br enables the reformation of a methyl group on the polymer chain end. Lastly, with the mechanistic insights in hand, we added exogenous bromine sources to increase the concentration of HBr and improved the acetophenone yield, becoming the major degradation product. We envision that our insights into the polystyrene degradation mechanism will further enable divergent product selectivity.
-
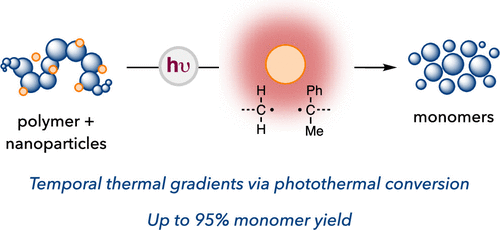
Photothermal Mediated Chemical Recycling to Monomers via Carbon Quantum Dots
J. Am. Chem. Soc. 2023, 145, 29, 16090–16097
Publication Abstract
Plastic recycling strategies to combat rapidly increasing waste buildup are of utmost environmental importance. Chemical recycling to monomers has emerged as a powerful strategy that enables infinite recyclability through depolymerization. However, methods for chemical recycling to monomers typically rely on bulk heating of polymers, which leads to unselective depolymerization in complex polymer mixtures and the formation of degradation byproducts. Here, we report a selective chemical recycling strategy facilitated by photothermal carbon quantum dots under visible light irradiation. Upon photoexcitation, we found that carbon quantum dots generate thermal gradients that induce depolymerization of various polymer classes, including commodity and postconsumer waste plastics, in a solvent-free system. This method also provides selective depolymerization in a mixture of polymers, not possible by bulk heating alone, enabled by localized photothermal heat gradients and the subsequent spatial control imparted over radical generation. Photothermal conversion by metal-free nanomaterials facilitates chemical recycling to monomers, an important approach in addressing the plastic waste crisis. More broadly, photothermal catalysis enables challenging C–C bond cleavages with the generality of heating but without indiscriminate side reactions typical of bulk thermolysis processes.
-
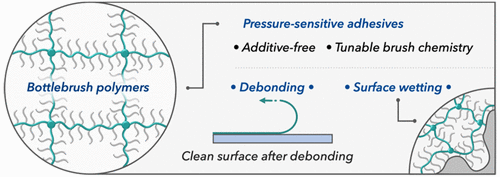
Sticky or Not: Adhesion by Architectural Design
ACS Cent. Sci. 2023, 9, 2, 134–136
Publication Abstract
That sticky residue left behind when a piece of electrical tape is removed─no more! In this issue of ACS Central Science, Dobrynin, Sheiko, and co-workers describe novel pressure-sensitive adhesives (PSAs) through the architectural engineering of bottlebrush polymers. These new adhesives are additive-free, meaning they do not leave behind a residue after debonding, typical of many commercial pressure-sensitive adhesives.
-
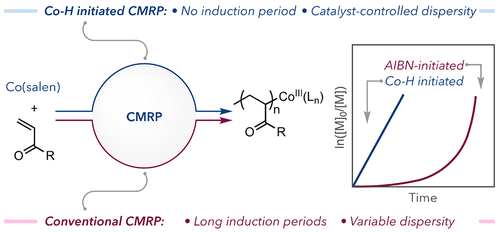
A Hydrometalation Initiation Mechanism via a Discrete Cobalt-Hydride for a Rapid and Controlled Radical Polymerization
J. Am. Chem. Soc. 2022, 144, 29, 13311–13318
Publication Abstract
Cobalt-mediated radical polymerization (CMRP) is a versatile technique for controlling the polymerization of vinyl monomers via reversible termination using CoII complexes as persistent radical deactivators. Here, we report a facile approach for the in situ generation of Co–H as a discrete initiator and mediator for CMRP of acrylate and acrylamide monomers, overcoming the limitations of existing initiation strategies. In situ oxidation of a CoII complex followed by transmetalation with silane generates a Co-H species, which initiates polymerization via hydrometalation of the monomer. This method precludes an induction period with excellent control over targeted molecular weight and dispersity. Strikingly, our approach allows complete polymerization when the induction period ends for conventional CMRP. A broad scope of monomers is amenable to this protocol, including acrylates and acrylamides. Tunable catalyst electronics afford tailored dispersity while maintaining agreement in molecular weight in stark contrast to conventional methods. Elimination of this induction period imbues polymerization behavior entirely to the catalyst electronic effects on reversible deactivation/activation rates.
-
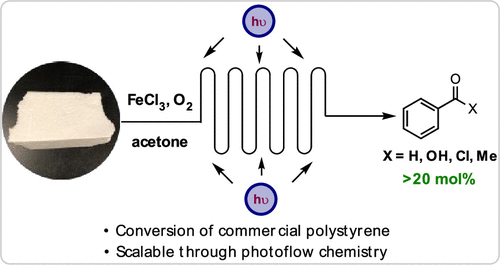
Chemical Upcycling of Commercial Polystyrene via Catalyst-Controlled Photooxidation
J. Am. Chem. Soc. 2022, 144, 13, 5745–5749
Publication Abstract
Chemical upcycling of polystyrene into targeted small molecules is desirable to reduce plastic pollution. Herein, we report the upcycling of polystyrene to benzoyl products, primarily benzoic acid, using a catalyst-controlled photooxidative degradation method. FeCl3 undergoes a homolytic cleavage upon irradiation with white light to generate a chlorine radical, abstracting an electron-rich hydrogen atom on the polymer backbone. Under the oxygen-rich environment, high MW polystyrene (>90 kg/mol) degrades down to <1 kg/mol and produces up to 23 mol % benzoyl products. A series of mechanistic studies showed that chlorine radicals promoted the degradation via hydrogen-atom abstraction. Commercial polystyrene degrades efficiently in our method, showing the compatibility of our system with polymer fillers. Finally, we demonstrated the potential of scaling up our approach in a photoflow process to convert gram quantities of PS to benzoic acid.

