Another strategy for polymer degradation being employed in the Stache lab is photothermal conversion mediated chemical recycling to monomer, wherein we leverage the photothermal effect exhibited by certain materials to generate intense heat gradients for at the surface of the excited agent. This heat, provided it is above the degradation and ceiling temperatures (Td and Tc, respectively) of the polymer, will efficiently induce depolymerization.
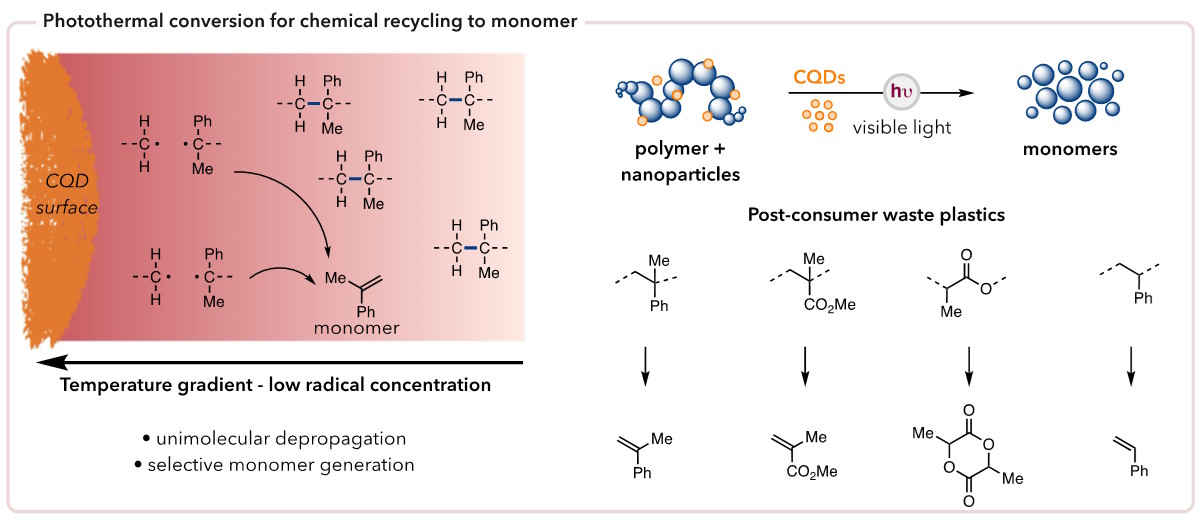
The creation of heat localized at the surface of the photothermal agent keeps reactive species confined to proximity, leading to lower rates of adverse side reactions.
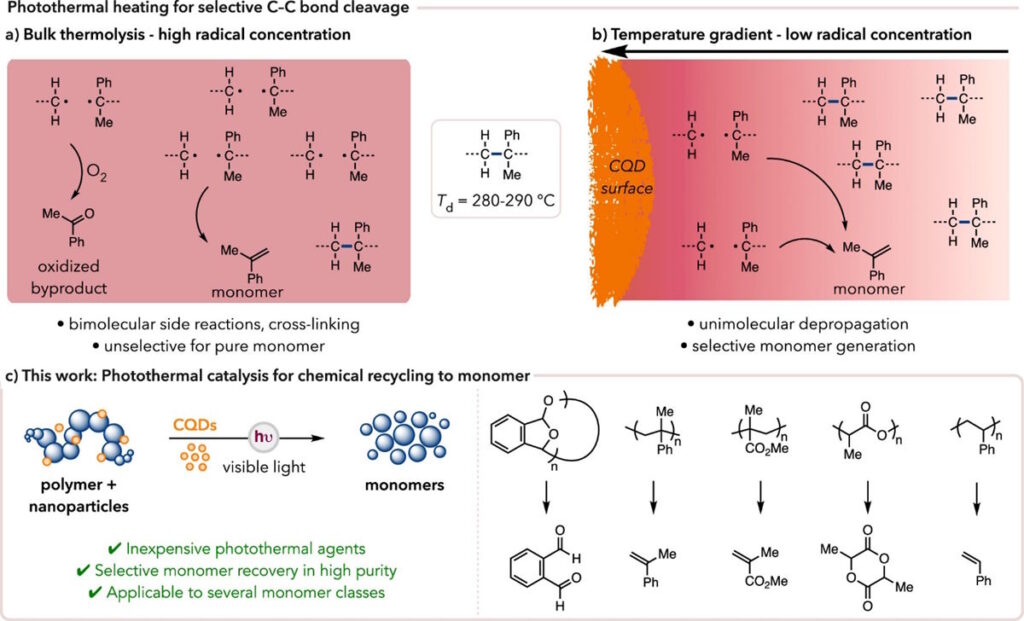
Photothermal conversion results when, upon light irradiation, the energy absorbed by a specific material or chemical relaxes vibrationally as opposed to by the traditional radiative pathways. One photothermal agent is carbon quantum dots (CQDs), which exhibit the same unique properties as traditional quantum dots but are made using inexpensive and readily available carbonaceous starting materials as opposed to heavy metals. For example, we selected L-ascorbic acid, or vitamin C, as our precursor. Utilizing this CQD, we were able to achieve 40-95% monomer recovery for a diverse array of relevant commercial plastics, including post-consumer waste.
