J. Am. Chem. Soc. 2023, 145, 37, 20311–20318
Article
Publication Abstract
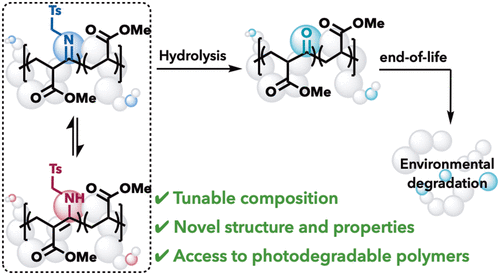
Installing ketones into a polymer backbone is a known method for introducing photodegradability into polymers; however, most current methods are limited to ethylene–carbon monoxide copolymerization. Here we use isocyanides in place of carbon monoxide in a copolymerization strategy to access degradable nonalternating poly(ketones) that either maintain or enhance the thermal properties. A cobalt-mediated radical polymerization of acrylates and isocyanides synthesizes nonalternating poly(acrylate-co-isocyanide) copolymers with tunable incorporation using monomer feed ratios. The kinetic product of the polymerization is a dynamic β-imine ester that tautomerizes to the β-enamine ester. Hydrolysis of this copolymer affords a third copolymer microstructure─the elusive nonalternating poly(ketone)─from a single copolymerization strategy. Analysis of the copolymer properties demonstrates tunable thermal properties with the degree of incorporation. Finally, we show that poly(acrylate-co-isocyanide) and poly(acrylate-co-ketone) are photodegradable with 390 nm light, enabling chain cleavage.