Advancing materials science and synthesis.
Based in the Frick Chemistry Laboratory at Princeton University, the Stache Lab integrates diverse chemistry disciplines, including organic chemistry, photochemistry, inorganic materials, and polymer chemistry, to pioneer advancements in materials science and synthesis.
Recent publications.
-
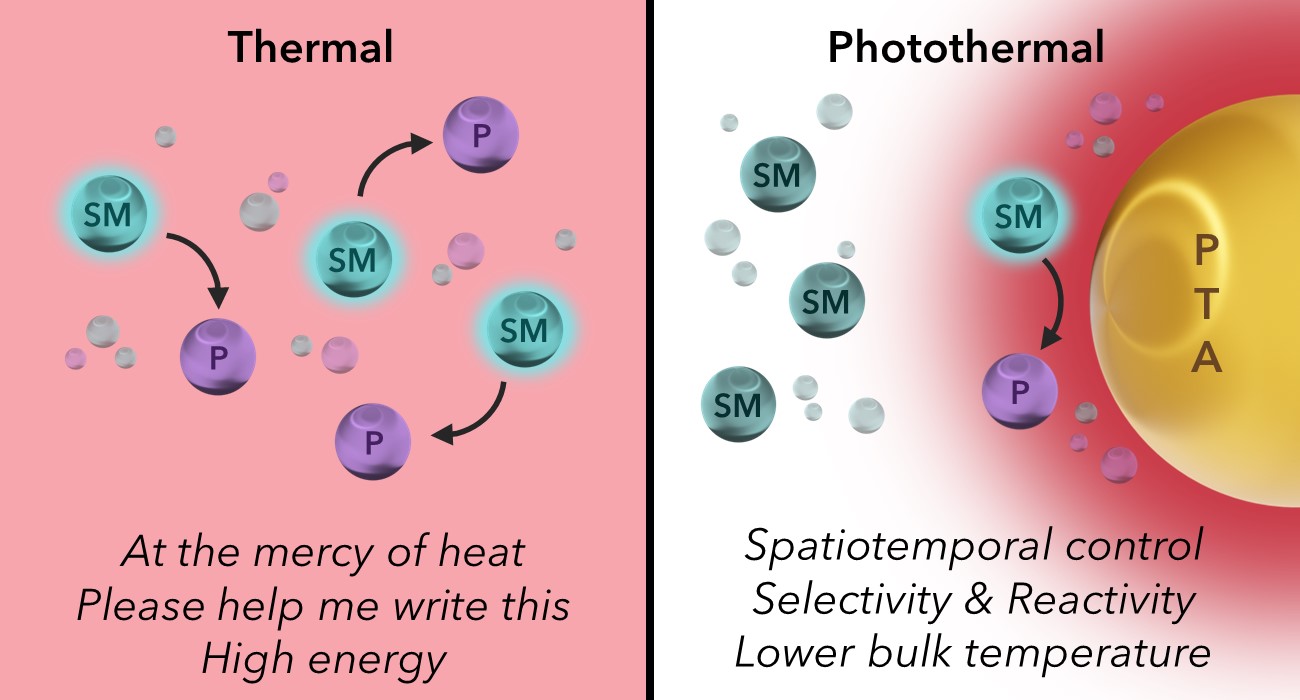
Recent Applications of Photothermal Conversion in Organic Synthesis
ACS Cent. Sci. 2024, 10, 8, 1460-1472.
Outlook
Publication Abstract
Photothermal conversion is a novel heating method that has emerged in recent years, wherein certain species can convert light to heat with great efficiency. These photothermal agents have shown immense promise for generating nanoscale thermal gradients under mild, visible light irradiation, providing a pathway for combining photochemistry with thermally driven reactivity. While this novel heating mechanism has been leveraged to great effect for applications such as photothermal therapeutics and steam water purification, it has seen limited use in organic synthesis. This outlook explores instances wherein the photothermal effect was used directly or as a synergistic component to drive organic reactions and postulates how it may be used moving forward.
-
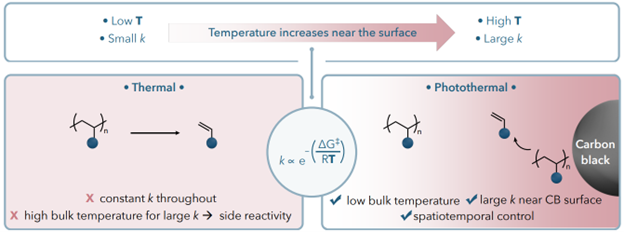
Photothermal Conversion Recycling of Commercial Polystyrene Plastic
Working Paper
Publication Abstract
Photothermal conversion can promote plastic depolymerization (chemical recycling) through light-to-heat conversion. The highly localized temperature gradient on photothermal agent surface allows selective heating with spatial controls not observed with bulk heating. However, identifying practical photothermal agents that are easily incorporated and reusable can be challenging. Interestingly, the rarely recycled black plastics containing carbon black is a potential candidate for photothermal conversion recycling. Herein, we use photothermal conversion to depolymerize commercial polystyrene plastics back into styrene monomers using the pigment in black plastics. Synthesized polystyrene-carbon black composites were depolymerized under white LED light irradiation, producing styrene monomer in up to 60 % yield. We demonstrate the recyclability of monomer and carbon black for a fully circular plastics economy. Ultimately, commercial black polystyrene samples are successfully converted to styrene through photothermal depolymerization without additional additives, with yields up to 70 % under focused solar irradiation in just 5 minutes. Broadly, this sustainable method holds the potential to actualize a closed-loop economy of black plastics.
-
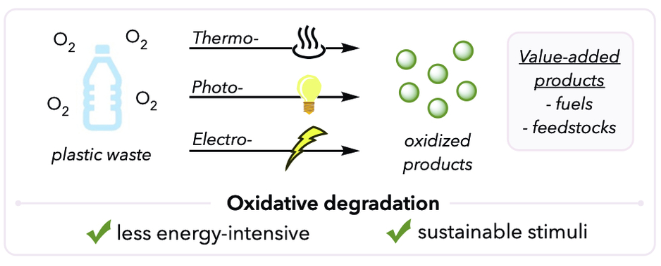
Recent advances in oxidative degradation of plastics
Chem. Soc. Rev. 2024, 53, 7309-7327
Tutorial Review
Publication Abstract
Oxidative degradation is a powerful method to degrade plastics into oligomers and small oxidized products. While thermal energy has been conventionally employed as an external stimulus, recent advances in photochemistry have enabled photocatalytic oxidative degradation of polymers under mild conditions. This tutorial review presents an overview of oxidative degradation, from its earliest examples to emerging strategies. This review briefly discusses the motivation and the development of thermal oxidative degradation of polymers with a focus on underlying mechanisms. Then, we will examine modern studies primarily relevant to catalytic thermal oxidative degradation and photocatalytic oxidative degradation. Lastly, we highlight some unique studies using unconventional approaches for oxidative polymer degradation, such as electrochemistry.

Our team.
The Stache Lab is led by Principal Investigator, Erin Stache. Our research team comprises a group of exceptional post-doctoral associates and graduate students.

